 |
|
CHRIS CODUTO/Arizona Daily Wildcat
|
Jim Hill, from Glendale, Ariz., shows off the tubes connecting the total artificial heart to "big blue," the machine that keeps the heart circulating blood by compressed air opening and closing valves in the heart.
|
|
|
By Natasha Bhuyan
Arizona Daily Wildcat
Monday, September 13, 2004
Print this
Artificial heart helps seriously ill while waiting for transplant
The CardioWest Total Artificial Heart, designed by a UA surgeon, will receive FDA approval this month, just weeks after the results of its nine-year study were published in the New England Journal of Medicine.
The CardioWest Total Artificial Heart, an air-driven, biventricular implant, is given to seriously ill patients who suffer from heart failure and are in need of a transplant. Although it cannot permanently replace the heart, the artificial heart assists survival while a patient awaits a transplant.
Dr. Jack G. Copeland, UA professor of surgery, led the UMC team that developed the artificial heart in 1993.
"FDA approval has been the primary goal," said Copeland, who is the co-director of the Sarver Heart Center at the UA College of Medicine. "It will be a great day for us... we're ecstatic."
Although an FDA panel announced in March that the heart is effective, Copeland said the official approval will occur this month.
"This is a significant milestone not only for the company and for patients and their families, but also a huge step in medical history," said Dr. Marvin J. Slepian, professor of medicine, director of interventional cardiology at UMC and CEO of SynCardia Systems Inc., a private company that owns the artificial heart.
The artificial heart has two artificial ventricles that fill and eject blood when compressed by air from an outside machine, which controls the patient's heart rate and monitors vitals.
The nine-year artificial heart study reported that patients who received the heart had a one-year survival rate of 70 percent, as opposed to the 31 percent survival rate for patients who did not receive a heart.
 |
|
CHRIS CODUTO / Arizona Daily Wildcat
|
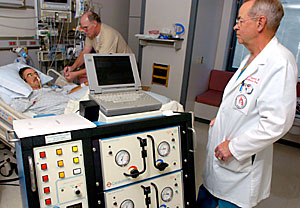
Dr. Jack G. Copeland monitors "big blue," the machine responsible for keeping the total artificial heart working in patients like Joe Cassles. Cassles, who suffers from coronary artery disease, received the heart Sept. 1 and says he feels much better with the heart.
|
|
|
Copeland said he originally began designing the artificial heart in the 1980s with the intention of creating a device that could permanently replace a person's heart.
Since its creation, 600 patients around the world have received an artificial heart. The longest a person has used the heart is 630 days.
Douglas McClellan, a senior clinical engineer who works with the artificial heart, said the FDA approval will result in increased use of the heart, and patients will soon be able to take a portable artificial heart home. Patients in France and Germany already have portable heart devices.
"They can haul it around like a piece of luggage," McClellan said.
McClellan said the success of the heart can be attributed to the fact that it is simple and reliable.
A regular human heart pumps five liters of blood per minute at rest and up to 20 liters per minute during vigorous exercise.
While other artificial devices pump four to five liters of blood per minute, the CardioWest heart can pump seven to nine liters per minute, which is more than any other implant able device in the world, Copeland said.
"This works better than anything else going," Copeland said.
The heart has been implanted in patients at UMC, Loyola Medical Center in Chicago, LDS Hospital in Salt Lake City, St. Luke's Medical Center in Milwaukee, University of Pittsburgh Medical Center, and in patients in European countries including Germany, Paris and Berlin.
Jim Hill, a UMC patient who has had an artificial heart for 167 days, said the device saved his life.
"They would have unplugged me and pulled a sheet over me 160-some days ago if it wasn't for this," said Hill.
 |
|
CHRIS CODUTO / Arizona Daily Wildcat
|
Dr. Jack G. Copeland, UA professor of surgery and co-director of the Sarver Heart Center at the UA College of Medicine, led the UMC team that developed the artificial heart in 1993. Copeland expects that the total artificial heart will gain FDA approval in the next month.
|
|
|
Hill, a resident of Glendale, Ariz, survived bypass surgeries and nine heart attacks. But five months ago, after another bypass surgery, Hill's heart stopped, and he went into a coma for 19 days.
When Hill awoke, his failed heart had been replaced with the CardioWest Total Artificial Heart.
"Now, I can do things just like a regular person," said Hill. "I'm in better shape than I was when I got here."
Hill said when he receives a heart transplant, his chance of survival will be 88 percent, compared to zero percent before he received the artificial heart.
Joe Cassles, a UMC patient who received the heart Sept. 1, said although being in a hospital is a difficult experience, he feels better with the artificial heart.
"I feel like it's really great to be alive instead of where I'd be without this machine," Cassles said.
Cassles, who suffered from coronary artery disease, said he was implanted with the heart after he almost died.
Upon its FDA approval, the heart will be used in 120 additional hospitals across the nation, according to FDA recommendations.
The heart will become more available to patients financially because Medicare and medical insurance companies will begin to cover the cost of the device, following the FDA approval, Copeland said.
Copeland said the artificial heart brings joy to everyone who works with it, including physicians, nurses and technicians.
"We really believe in this device," Copeland said. "It helps patients, it saves lives."
